Best Botox® Cosmetic

-
What is BOTOX® Cosmetic?
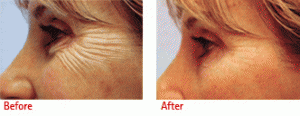
BOTOX® Cosmetic is a simple, non-surgical, physician-administered treatment that can temporarily smooth moderate to severe frown lines between the brows in people from 18 to 65 years of age. It is the only treatment of its type approved by the Food and Drug Administration (FDA). Results may vary but the effects can last up to 4 months.
BOTOX® Cosmetic is a purified protein produced by the Clostridium botulinum bacterium, which reduces the activity of the muscles that cause those frown lines between the brows to form over time.
BOTOX® Cosmetic is a purified protein produced by the Clostridium botulinum bacterium, which reduces the activity of the muscles that cause those frown lines between the brows to form over time.
Dr Byun will determine exactly where to administer the injections to achieve the best results. No anesthesia is required, although he may choose to numb the area with a cold pack or anesthetic cream prior to injecting. The entire procedure takes approximately tem minutes. Discomfort is minimal and brief. Most patients compare the sensation to a bug bite. You may resume normal activity immediately.
Radiesse™ by Bioform®
Radiesse is a synthetic injectable implant composed of smooth CaHA microspheres, that have a diameter range of 25m to 45m, suspended in an aqueous gel carrier. These consistently shaped and sized particles have proven safe and biocompatible while allowing gradual tissue ingrowth. The three applications which Radiesse has been cleared for in the United States are listed below. Additional indications are in various stages of development.
Restylane®
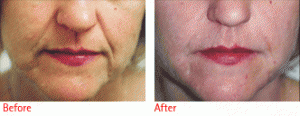
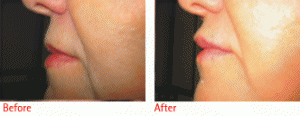
Restylane is a cosmetic dermal filler made of hyaluronic acid, a natural substance that already exists in the human body. The hyaluronic acid in Restylane is a crystal-clear gel called NASHA or Non-Animal Stabilized Hyaluronic Acid. NASHA is completely biocompatible with human hyaluronic acid.
Restylane maintains its shape using the body’s own moisture. The hyaluronic acid in Restylane is hydrophilic or “water loving.” As hyaluronic acid gradually degrades, each molecule binds to more water and over time, the same volume can be maintained with less hyaluronic acid. This ability of hyaluronic acid to bind to water is what helps provide lasting results.
Unlike rooster-derived hyaluronic acids and bovine collagen products, Restylane is free from animal proteins. This limits any risk of animal-based disease transmissions or development of allergic reactions to animal proteins.
After your treatment, you might have some redness or swelling. This will normally last less than seven days. Sunbathing and cold outdoor activities should be avoided until any redness or swelling disappear. If you are pregnant, breastfeeding, or under 18, you shouldn’t use Restylane.
Restylane is injected directly into the skin in tiny amounts by an ultrafine needle, resulting in minimal discomfort. The procedure is simple and convenient and results are practically instantaneous. To optimize your comfort during the short procedure, your physician may decide to numb the treatment area.
Restylane has a highly favorable safety profile. It’s composed of Non-Animal Stabilized hyaluronic acid. Hyaluronic acid is a substance found naturally in the human body. Restylane is fully biocompatible. There is limited risk of animal-based disease transmission or development of allergic reactions to animal proteins. No allergy testing is required before use.*
After your treatment, you might have some redness or swelling. This will normally last less than seven days. Sunbathing and cold outdoor activities should be avoided until any redness or swelling disappear. If you are pregnant, breastfeeding, or under 18, you shouldn’t use Restylane.
- The safety of Restylane for use during pregnancy, in breastfeeding females or in patients under 18 years has not been established.
- The safety of Restylane in patients with increased susceptibility to keloid formation and hypertrophic scarring has not been studied. Restylane should not be used in patients with known susceptibility to keloid formation or hypertrophic scarring.
- The patient should be informed that he or she should minimize exposure of the treated area to excessive sun and UV lamp exposure and extreme cold weather until any initial swelling and redness has resolved.
- If laser treatment, chemical peeling or any other procedure based on active dermal response is considered after treatment with Restylane there is a possible risk of eliciting an inflammatory reaction at the implant site. This also applies if Restylane is administered before the skin has healed completely after such a procedure.
Restylane is different than other hyaluronic acid-based products and bovine collagen.
Unlike rooster-derived hyaluronic acids and bovine collagen products, Restylane is free from animal proteins. This limits any risk of animal-based disease transmissions or development of allergic reactions to animal proteins.* Bovine collagen is derived from cowhide and requires an allergy test. Restylane can be administered without pretesting, so no waiting is required.
- Please observe the following after treatment with Restylane.
After your treatment, you might have some redness or swelling. This will normally last less than seven days. Sunbathing and cold outdoor activities should be avoided until any redness or swelling disappear. If you are pregnant, breastfeeding, or under 18, you shouldn’t use Restylane. - Avoid touching the treated area within six hours following treatment. After that, the area can be gently washed. Sunbathing and cold outdoor activities should be avoided until any redness or swelling disappear.
- If you have previously suffered from facial cold sores, there is a risk that the needle punctures could contribute to another recurrence. Speak to your physician about medications that may minimize a recurrence.
- Avoid exercise and alcohol for six hours after your treatment.
- Having a follow-up treatment before the product has fully dissipated may enhance the lasting effect. Please be sure to consult your physician about recommendations for touch-up or follow-up treatments.
- One week prior to your next treatment with Restylane, avoid taking St. John’s Wort, high doses of Vitamin E supplements, aspirin, and other non-steroidal anti-inflammatory medications, such as ibuprofen. These agents may increase bruising and bleeding at the injection site.